Which Statement Best Describes an Ionic Bond
1 How the bond between two oxygen atoms is formed. Ionic bonds form only between metals and nonmetals.

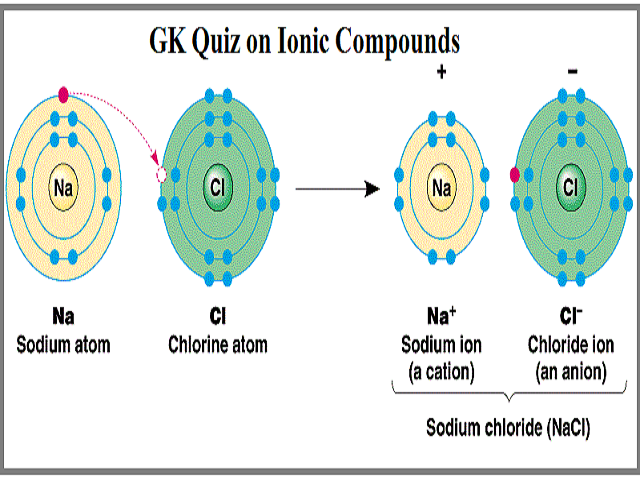
Ionic Compounds Bonds Structure Properties 1 6 5 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams
The transfer of electrons forms strong bods between ions.

. 8 How do atoms form bonds. 2 What is the bond between 2 oxygen atoms. Which statement best describes how an ionic bond forms.
You might be interested in. 7 Why do atoms form ionic bonds. It is insoluble in water.
Thats because metals want to give up electrons and nonmetals want to gain electrons. 11 What part of atom makes bonds. A force that holds two oppositely charged ions together.
One atom pulls an electron from another atom. Iteru 24K 1 year ago. It conducts electricity when molten.
Make sure you list the elements how many. D An electrostatic attraction exists between lithium ions and fluoride ions. B A lithium atom shares on electron with a fluorine atom C An electron is transferred from a lithium atom to a fluorine atom.
Which statement best describes an ionic bond. An ionic bond involves a metal that transfers one or more electrons to a nonmetal. Which statement best describes ionic bonding in lithium fluoride.
5 How would you describe the bond that exists between two oxygen atoms to make. An ionic bond involves two metals that exchange electrons. Two atoms attain equal electronegativities.
A chemical bond refers to the forces of attraction that exist between ions crystals atoms or molecules and as such are typically responsible for the formation of new chemical compounds. An ionic bond is the force of attraction that holds together positive and negative ions. 12 Which of the following is the best reason why atoms tend to combine with other atoms instead of existing alone.
Some properties of ionic compounds are high melting points. Which property best indicates that a compound contains an ionic bond. 13 What is chemical.
10 Which statement explains why the bonds between nonmetals tend to be covalent. It takes energy to remove valence electrons from an atom and form a positive ion. An ionic bond involves a metal that shares electrons with a nonmetal.
9 Why do atoms want to be stable. 4 Which answer below best describes the type of bond occurs between two oxygen atoms. An ionic bond involves a metal that transfers one or more electrons to a nonmetal.
Which statement explains the relationship between the amount of energy it takes to break a bond and the amount of energy released when the same bond is formed. It conducts electricity when molten. Which of the following happens when an ionic bond is formed.
What statement best describes the properties of ionic compounds. There is not a statement available so it is difficult to answer this. The statement which best describes an ionic bond is.
An ionic bond involves two nonmetals that share electrons. It has a low boiling point. A The positive and negative charges of the ions cancel out.
Which property best indicates that a compound contains an ionic bond. 3 What term describes the bond between atoms in a molecule of oxygen. Determine the number of atoms in the following chemical formulas.
It can be easily flattened.

Question Video Selecting The Statement That Does Not Describe Ionic Bonding Nagwa
Comments
Post a Comment